Carmel Company Moving Potential Hepatitis B Cure Into Clinical Trials
 The company's second lead platform is a microbiome technology
The company's second lead platform is a microbiome technology
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowA Carmel-based company believes it’s within months of having a potential cure for the hepatitis B virus in clinical trials—potentially giving millions of people in the U.S. a treatment for a virus that can be deadly. Assembly Biosciences, Inc. says the impact could be even greater internationally. The virus, which is the leading cause of chronic liver disease and liver transplants, infects up to 300 million people worldwide, with 90 million of those patients in China alone.
“There’s a huge population of chronically-infected patients; these patients go on to many types of disorders that cause somewhere between 500,000 and one million deaths each year,” says Assembly Biosciences President and Chief Executive Officer Derek Small. “It’s a very serious disease.”
Small says most scientists have attempted to cure the disease by targeting the virus, which exists in the liver. However, Assembly Biosciences says it uses a different strategy by targeting an HBV core protein within the virus’ DNA; the unique approach is based on discoveries made by Indiana University Molecular and Cellular Biochemistry Professor Dr. Adam Zlotnick.
“We have the ability to target HBV like nobody else has had to date,” says Small, “and now we have three different drugs in development for a combination to cure HBV.”
The company started in 2012 as Assembly Pharmaceuticals, and after collecting $1.5 million in seed funding, its leaders soon realized the resources of a larger company would speed the pace of commercialization. In 2014, it merged with New York-based Ventrus Biosciences, a public firm that provided a $25 million cash infusion for the HBV platform, and a new company was born: Assembly Biosciences.
The merger also meant Assembly Biosciences now had two lead platforms: the HBV technology, as well as a microbiome therapeutic, which was Ventrus’ area of expertise. While Small acknowledges it’s unusual for a single company to have two unrelated platforms, he says it’s been good for business.
“When we merged the company two years ago, both [platforms] were very pre-clinical. Now, both will be going into the clinic within the next few months,” says Small. “We believe by end of year, the HBV [drug] will be in the clinic, and shortly after, we’ll have the microbiome platform in the clinic as well.”
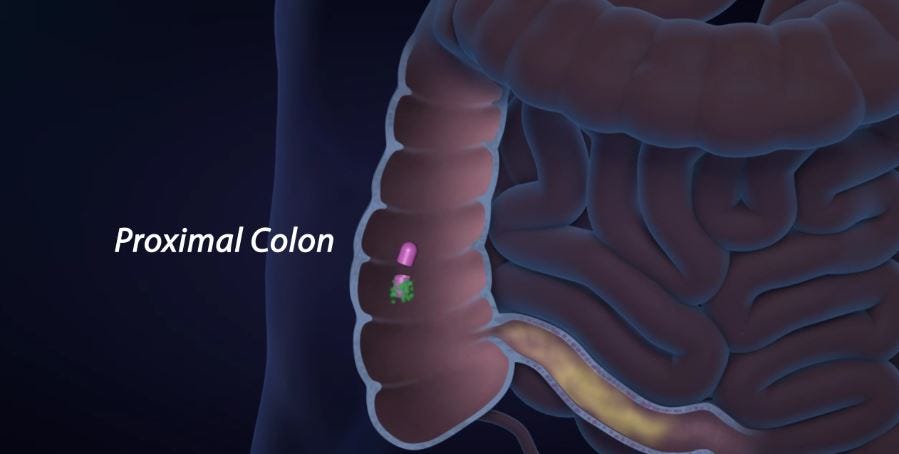
The microbiome technology is expected to provide a treatment for Clostridium difficile infection (CDI), but could also lead to therapeutics for inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). Small says such problems occur when “good” bacteria that naturally exist in our gut “get out of whack.”
The current standard for treating CDI is fecal material transplant (FMT); feces are processed to obtain the main bacteria ingredients, then delivered directly to the colon through a gastric tube inserted in the nose or a colonoscopy. While Small says FMTs have “profound efficacy,” they’re also labor-intensive, invasive and have a high “ick” factor. However, FMTs deliver treatment directly to the lower GI tract; a difficult destination for any treatment because the harsh environment of the stomach, small and large intestines are designed to “sterilize” everything that’s digested.
Assembly Biosciences believes it has developed a more convenient method to deliver the same good bacteria to the colon. Rather than processing feces, Assembly Biosciences manufactures the same bacterial strains and encapsulates them in a proprietary pill technology called Gemicel. The pH level of the pill’s target region—such as the colon—triggers the capsule’s shell to dissolve and release the drug. Gemicel capsules also have inner and outer layers that can be engineered to dissolve at different pH levels, making it possible to deliver two different therapeutics to two different locations.
Small says Assembly Biosciences has worked hard to build the “right team” for each technology, “and now it’s just time to execute.”
“When you start to get an understanding of what your work can do to help patients’ lives, it changes the way [the team] thinks and its motivations,” says Small. “It helps align the whole company to work harder, be smarter and cut to the chase to really achieve our goals, which is getting these drugs to help the patients.”
Small says Gemicel is the answer to the longstanding problem of delivering drugs to the lower GI tract.
Small says after recent hires this fall, Assembly Biosciences has completed its team and is ready to execute each platform’s strategy.

