‘Light Up’ Cancer Technology to Enter Phase 3 Trials
 On Target says its technology "lights up" cancerous tissue
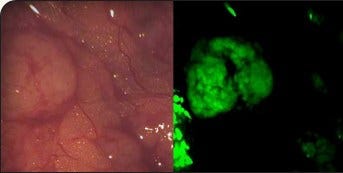
On Target says its technology "lights up" cancerous tissue
Subscriber Benefit
As a subscriber you can listen to articles at work, in the car, or while you work out. Subscribe NowAn Indiana startup believes it will soon be lighting the way to improved cancer treatment. Buoyed by “tremendous success” in recent clinical trials, Purdue Research Park-based On Target Laboratories is developing a technology that illuminates cancer cells during surgery, so doctors can see exactly where the disease is and remove it more easily. Company leaders believe these glowing guideposts—now entering Phase 3 clinical trials for ovarian cancer—will bring certainty to the treatment of a disease that is notoriously elusive.
“You inject our technology into a patient who has a specific cancer two hours before surgery, and it clears the rest of the body except for where the tumor is,” says On Target Chief Executive Officer Marty Low. “It lights up the tumor, so the surgeon…can identify the tumor; there’s a lighted roadmap, because it fluoresces. In the trials we’ve done, we’ve had tremendous success.”
With about $32 million in funding so far, including a recent $5.2 million shot in the arm from private investors, the company will use the money to advance the technology; it will begin Phase 3 clinical trials for OTL38, its lead molecule, by the end of the year. More than 200 patients were part of Phase 2 clinical trials testing the use of OTL38 in ovarian cancer, a disease where the technology could be especially helpful for surgeons.
“By the time a woman finds out she has [ovarian cancer], it’s basically spread up into her stomach area, so most of the surgeries entail cutting open the whole stomach region and spreading the entire abdomen open to find the lesions or tumor,” says Low. “Unlike other tumors, it’s not a solid mass; there are many lesions often spread throughout the whole stomach area—which is very bad. It’s like stars in the sky: a millimeter here, there and everywhere.”
In fact, the Ovarian Cancer National Alliance says advanced stage ovarian cancer has a 70 percent recurrence rate, often because the surgeon can’t see all of the cancer during the initial operation.
Low says, currently, doctors find cancerous lesions by sight and touch, so patients are reliant on the doctor’s expertise. OTL38 illuminates cancer cells as small as one-thousandth of a millimeter, enabling surgeons to find lesions that even a microscope would miss.
Noting “the name of the game is data,” Low says OTL38 had near-perfect results with regard to sensitivity in the Phase 2 clinical trial focused on ovarian cancer.
“98 percent of the time in our study, if it was cancer, it lit up—so almost 100 percent of the time. The other side is, if it lights up, it better be cancer, so you don’t have any false positives. 96 percent of the time, if it lit up, indeed, it was cancer,” says Low. “That data is extremely good. There’s no other marker out there that we know of that will identify it that accurately.”
On Target says the technology is also showing great promise for lung cancer; the company will begin a Phase 2 clinical trial focused on that disease in August. Low says, in addition to benefitting patients’ prognosis, OTL38 can cut treatment costs—especially for patients with lung cancer—by reducing their time on the operating table.
“With lung cancer, you usually have one large lesion or tumor. [Surgeons] will often take that tumor out, and they have to send it to pathology. Pathology can take, on average, 20 to 25 minutes to come back with a diagnosis,” says Low. “In the OR, if they use our technology, we can identify the cancer in real time. You think about saving 20 minutes in an OR, where the costs range anywhere from $50 to $300 per minute with staff, anesthesia, the room—it’s a considerable savings in costs.”
On Target has also launched investigator-sponsored trials for renal, endometrial and pituitary cancers; the technology must be separately approved by the U.S. Food and Drug Administration for its use with each form of cancer.
Company leaders believe the technology itself—aiming for commercialization in 2018—won’t be costly to implement, requiring only the OTL38 molecule and the “camera” device that visualizes the “glow in the dark” effect. On Target says the technology will give immediate feedback on the operating table—a priceless benefit that provides certainty for doctors and peace of mind for patients.
Low says surgeons described OTL38 as a “home run,” especially after a patient has had chemotherapy.
Low says On Target has built a network with leading device companies and medical centers throughout the world to bring OTL38 to market.

